could u explain this in more detain please 

Chlorine has two isotopes, 35Cl and 37Cl, in the approximate ratio of 3 atoms of 35Cl to 1 atom of 37Cl. You might suppose that the mass spectrum would look like this:
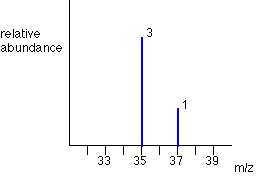
The problem is that chlorine consists of molecules, not individual atoms. When chlorine is passed into the ionisation chamber, an electron is knocked off the molecule to give a molecular ion, Cl2+. These ions won't be particularly stable, and some will fall apart to give a chlorine atom and a Cl+ ion. The term for this is fragmentation.
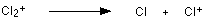
If the Cl atom formed isn't then ionised in the ionisation chamber, it simply gets lost in the machine - neither accelerated nor deflected.
The Cl+ ions will pass through the machine and will give lines at 35 and 37, depending on the isotope and you would get exactly the pattern in the last diagram. The problem is that you will also record lines for the unfragmented Cl2+ ions.
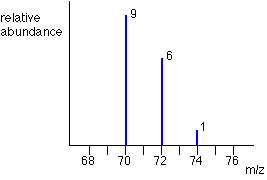
Think about the possible combinations of chlorine-35 and chlorine-37 atoms in a Cl2+ ion.
Both atoms could be 35Cl, both atoms could be 37Cl, or you could have one of each sort. That would give you total masses of the Cl2+ ion of:
35 + 35 = 70
35 + 37 = 72
37 + 37 = 74
That means that you would get a set of lines in the m/z = 70 region looking like this:
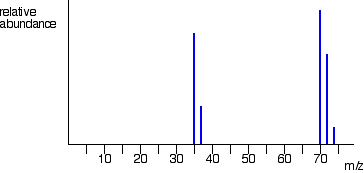
The overall mass spectrum looks like this:
yaa?