I'm stuck in june 2005 Q7,10,11,31,32
Nov.2002 Q8, 11, 39. 


so, if anyone can help me asap i shall be thankful.
I'll only answer those that ashish did not.
 Jun 0510.
Jun 0510. The forward reaction is endothermic as indicated by the positive enthalpy change.
Hence according to Le Chatelier's principle, an increase in temperature will only favour the forward reaction in order to oppose the change. This will result in an increase in pressure since there will be two moles of NO
2 gas formed at the expense of only one mole of N
2O
4 gas.
An increase in pressure only occurs when the volume increases or when the vessel's volume decreases. In this case the volume of the mixture will increase. From here we can already eliminate
C and
D.
A gas always expand when temperature is increased. Expansion also implies an increase in volume.
Hence answer is
B32. We'll proceed by elimination.
1.
The mass of Y used in the experiment was 
---> That's true since even as temperature continues to rise, the mass of vapour does not increase further showing that all the solid has turned into vapour.
2.
The pressure of the vapour was constant for all the temperatures above 
. ----> That is not the case. Increase in temperature leads to expansion. This will cause the volume as well as the pressure to rise.
3.
Liquid appeared at temperature 
-----> It may be possible but there is nothing on this graph which indicates it was the case. So we cannot take it for granted.

Therefore answer is
DNov 0211. K
p =
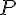
(H
2).
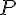
(I
2) /
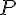
(HI)
2 at equilibrium.
It has been given that at equilibrium

mole of HI has been dissociated. This means that (
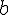
-

) moles is left. As for the number of moles of products; H
2 =

/2 and I
2 =

/2
Hence K
p = (

/2).(

/2) / (
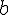
-

)
2This ends up to K
p =
 2
2 / 4(
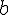
-

)
2Hence answer is
D39. Ethanol dissociates to form the corresponding ions as shown :
CH
3CH
2OH <=> CH
3CH
2O
- + H
+When H
2SO
4 is added, there is an increase in H
+ ions. Hence according to Le Chatelier's principle, the equilibrium will shift to the left forming more ethanol at the expense of its corresponding ions.
Hence the ions present will be mainly HSO
4- and CH
3CH
2O
+H
2 as H
+ combines with ethanol.
The first one is rejected since it will have the tendency to form ethanol and will not be present.
Hence answer is
C.
Hope it helps
