plz can u help me in ppr 1 Nov.03 Q2,6,13,31,40. thanx in advance 
2. First you need to find the % of phosphorus in P
2O
5Mr of P
2O
5 = 2(31) + 5(16) = 142
Hence % of phosphorus in P
2O
5 = 2(31)/142 x 100 = 43.6%
Since P
2O
5 forms part of only 30% of the fertiliser, % of phosphorus in P
2O
5 in the fertiliser will be (30/100 x 43.6) =
13.1%Answer is
B6. That's kind of obvious.

Six bonding electrons imply a molecule which is composed of only 3 bonds since a bond carries 2 electrons.
A C2H4 ------> 6 bonds (12 bonding electrons)
B C2F6 ------> 7 bonds (14 e)
C H2O -------> 2 bonds (4 e)
D NF3 -------> 3 bonds (6 e)
NOTE : A
double bond carries 2 pairs of electrons.
Hence Answer is
D13. You should be aware of the trends of ionisation energies for period 3. Take a look
here.
There is a fall at Aluminium since there is a change in sub-shells namely from 3s to 3p. That's why aluminium has a lower 1st I.E.

Answer is
B31. So let's examine the answers one by one.
1.
An acid- base reaction : That's true since SO
3 and water combines to form sulfuric acid. Sulfuric acid then reacts with basic NH
3 to form the corresponding salt.

2.
Ionic bond formation : That's true as well. The salt exists in the form of NH
4+ and SO
42- which are ions involved in ionic bonds.
3.
Oxidation and reduction : You need to calculate the oxidation numbers of the different elements in order to determine this.
If we just look at the equation, we might have the impression that SO
3 is being oxidised but this is not the case.
Let oxidation number of sulfur in SO
3 be
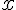
and take oxidation number of O as -2.
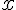
+ 3(-2) = 0 ---->
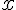
= +6
Now for SO
42-.
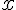
+ 4(-2) = -2 ---->
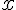
= +6
Since oxidation number remains the same, there has been neither oxidation nor reduction. I'll let you calculate the oxidation numbers of the other elements yourself for confirmation.

Hence answer is
B since only 1 and 2 are good.
40. Heating under reflux with NaOH is an alkaline hydrolysis, i.e there is the breaking of bonds usually COO.
Therefore the ester bond will be broken and two compounds, namely an alcohol and a carboxylic acid will be formed.
Since NaOH is used the carboxylate will be formed instead of the carboxylic acid, but upon distillation the carboxylate will get back to the acid.
Product 3 cannot be formed since it involves the breaking of a C=O which is not possible.
Hence answer is
D.
Hope it helps
