Thanks to answer me Deadly_king,,
Ok but why the propanol structure is not like this;
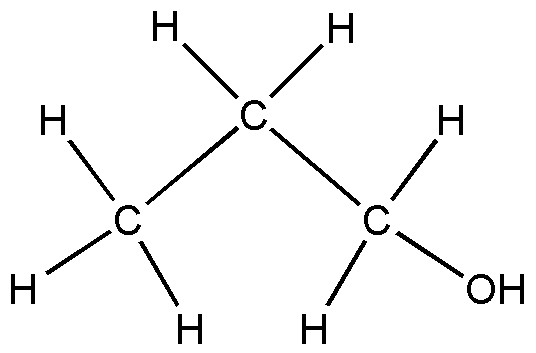
c=c-c- ( is an isomer)
 ??
??
of
this
c-c-c
Sorry dude.......C is not propanol. It is propen-1-ol like Garfield said

I described B instead of C.

An
isomer is a compound with same molecular formula but different structural formula.
A compound consisting a double bond (C=C) will
NOT have the same formula as that of hydrocarbon(C-C).
Example : Propene and propane
Propene ----> H
2C=CHCH
3Propane ----> H
3CCH
2CH
3You will note that Propane has two hydrogen atoms more than propene. Hence they are not isomers

By the way.......thanks for correcting me Garfield
