For extraction of iron, I shall post a picture from my textbook - explains it really well, after my scanner or even the webcam decide to function.


OK not working out really well so here are the notes :
Extraction of iron.Iron ore --->
haematite (Iron (II) Oxide - Fe2O3)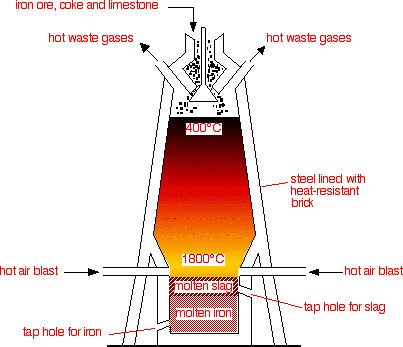
[If image too small - Right-click > View image]
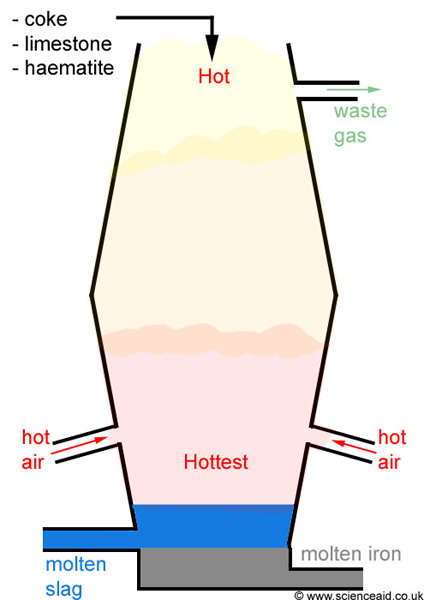
yeah so as you can see the extraction of Fe takes place in a blast furnace.
Raw materials used to put at the top.>Iron ore (Fe
2O
3)
>Coke (Main source for Carbon needed for the reduction process)
>Limestone (needed to remove the impurities in the form of 'slag')
Steps in which the extraction is conducted :1. Iron ore, coke and limestone are fed into the top of the blast furnace.
2. Hot air blasted up the furnace from the bottom.
3. Oxygen from the sir reacts with coke to from carbon dioxide.
C (s) + O2 (g) ---> CO2 (g)4.Carbon dioxide reacts with more coke to form carbon monoxide
CO2 (g) + C (s) ---> 2CO (g)5.Carbon monoxide is a reducing agent.
Iron (II) oxide is reduced to iron :
Fe2O3 (s) + 3CO (g) ---> 2Fe (l) + 3CO2 (g)6. Dense molten iron runs to the bottom of the furnace and is run off. There are many impurities in iron ore. The limestone helps to remove these shown in steps 7 and 8.
7. Limestone is broken down by heat to calcium oxide :
CaCO3 (s) ---> CaO (s) + CO2 (g)8. Calcium oxide reacts with impurities like sand (silicon dioxide) to form a liquid called 'slag':
CaO (s) + SiO2 (g) ---> CaSiO3 (l) (calcium silicate)
the liquid slag falls to the bottom of the furnace and is tapped off.
The overall reaction :2Fe2O3 (s) + 3C ---> 4Fe + 3CO2Reduction reaction stages :
Stage 1 - The coke reacts with oxygen blasted into the furnace :C (s) + O2 (g) ---> CO2 (g)Stage 2 - The carbon dioxide is reduced by unreacted coke to form carbon monoxide :CO2 (g) + C (s) ---> 2CO (g)
Stage 3 - The iron (iii) oxide is reduced by the carbon monoxide to iron :Fe2O3 (s) + 3CO (g) ---> 2Fe (s) + 3CO2 (g)