Q4) C
H has 1
C has 6
O has 8 (times 3)
and the overall charge is -1 (which means there's one more electron)
1+6+24+1=32
Q9) A
just the reverse right....so greater activation energy (endothermic reaction) and so
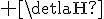
Q13) Phosphoric acid is more acidic as compared to the others. D
Q19) ammonium ion acts as an acid ( proton donor) H+ ion donor. hence D
Q39) 2nd one is an isomer of the first...so it's absolutely ok.
Q32) water is amphoteric. it can act as an acid and also a base. It can donate H+ ions and can also accept them forming(hydronium ion)
Q26) count the no of Carbon atoms in the product formed...lesser than the one they are asking for
Q29) again count the no of carbon atoms. In A the C from CN is not present. The count of C atoms should increase by 1 due to addtion of CN- ion